A method to study bladder urothelial cellular function in situ
Main Article Content
Keywords
urothelium, urothelial cells, potassium current
Abstract
Objective: The bladder urothelium is comprised of basal, intermediate, and apical urothelial cells. Ability to functionally study an individual urothelial cell while preserving its in situ location would represent an advance in bladder urothelial biology.
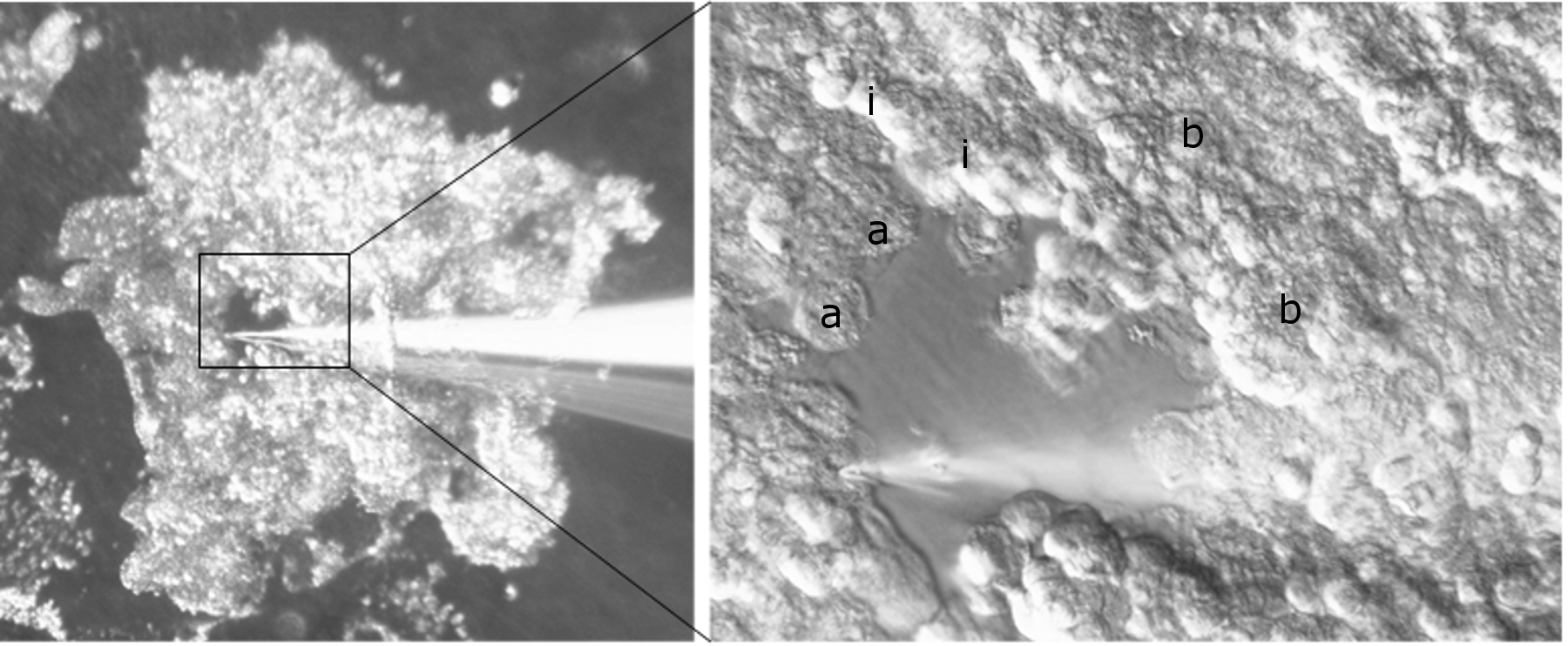
Materials and Methods: Mice were euthanized and cardiac perfused with phosphate-buffered saline. Bladders were then excised. Urothelial sheets were dissected off the lamina propria using forceps with a microscope (5x magnification). Sheets were stained with H&E. In separate experiments, cell-attached electrophysiology was performed by placing urothelial sheets in Ringer’s bath solution, with either basal or apical surface down. Using 40x magnification, individual urothelial cells from different layers were identified. Potassium currents on these cells were measured in situ using single channel patch-clamp technique.
Results: Histology revealed that urothelial sheets were free of lamina propria and smooth muscles. The proportion of apical cells, compared to proportion of intermediate and basal cells, with measureable potassium currents was considerably higher in apical cells (69% of apical versus 16% of intermediate and 18% of basal cells). Of the active patches detected in apical cells, 100% of these patches showed a 43 pS current conductance. For intermediate and basal cells, 75-83% demonstrated a 43 pS current and 17-25% demonstrated a 22 pS current. Single cells, from all 3 layers, could also be individually microdissected completely off the urothelium.
Conclusions: A novel approach was developed in which individual urothelial cells within the multi-layered urothelium were identified and functionally studied in situ. Electrophysiologic characterization revealed different phenotype between the cells from different layers. Single cells from an identified layer can be harvested off the urothelium allowing for other studies. This technique allows investigators to study various cellular functions while preserving cellular location within the urothelium.
Metrics
References
2. Sun TT, Zhao H, Provet J, Aebi U, Wu XR (1996) Formation of asymmetric unit membrane during urothelial differentiation. Mol Biol Rep 23:3-11.
3. Carattino MD, Prakasam HS, Ruiz WG, Clayton DR, McGuire M, et al (2013) Bladder filling and voiding affect umbrella cell tight junction organization and function. Am J Physiol Renal Physiol 305:F1158-68.
4. Birder L, Wyndaele JJ (2013) From urothelial signalling to experiencing a sensation related to the urinary bladder. Acta Physiol (Oxf) 207:34-9.
5. Bäckhed F, Söderhäll M, Ekman P, Normark S, Richter-Dahlfors A (2001) Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell Microbiol 3:153-8.
6. Sun TT (2006) Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol 291(1):F9-21.
7. Signoretti S, Pires MM, Lindauer M, Horner JW, Grisanzio C, et al (2005) p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci USA 102:11355-60.
8. Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, et al. (2002) Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283:F242-53.
9. Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, et al. (2004) Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287:F305-18.
10. Yu J, Manabe M, Wu XR, Xu C, Surya B, Sun TT (1990) Uroplakin I: a 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J Cell Biol 111:1207-16.
11. Yu W, Hill WG (2011) Defining protein expression in the urothelium: a problem of more than transitional interest. Am J Physiol Renal Physiol 301:F932-42.
12. Spector DA, Deng J, Stewart KJ (2013) Hydration status affects sodium, potassium, and chloride transport across rat urothelia. Am J Physiol Renal Physiol 305:F1669-79.
13. Sun Y, Chen M, Lowentritt BH, Van Zijl PS, Koch KR, et al. (2007) EGF and HB-EGF modulate inward potassium current in human bladder urothelial cells from normal and interstitial cystitis patients. Am J Physiol Cell Physiol 292:C106-14.
14. Li M, Sun Y, Simard JM, Wang JY, Chai TC (2009) Augmented bladder urothelial polyamine signaling and block of BK channel in the pathophysiology of overactive bladder syndrome. Am J Physiol Cell Physiol 297:C1445-51.
15. Kullmann FA, Shah MA, Birder LA, de Groat WC (2009) Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296:F892-901.
16. Li M, Sun Y, Simard JM, Chai TC (2011) Increased transient receptor potential vanilloid type 1 (TRPV1) signaling in idiopathic overactive bladder urothelial cells. Neurourol Urodyn 30:606-11.
17. Li M, Sun Y, Tomiya N, Hsu Y, Chai TC 2 (2013) Elevated polyamines in urothelial cells from OAB subjects mediate oxotremorine-evoked rapid intracellular calcium rise and delayed acetylcholine release. Am J Physiol Renal Physiol 305:F445-50.
18. Gupta GN, Lu SG, Gold MS, Chai TC (2009) Bladder urothelial cells from patients with interstitial cystitis have an increased sensitivity to carbachol. Neurourol Urodyn 28:1022-7.
19. Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA (2008) Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol 294:F971-81.
20. Wu C, Gui GP, Fry CH (2011) Intracellular Ca(2+) regulation and electrophysiolgical properties of bladder urothelium subjected to stretch and exogenous agonists. Cell Calcium 49:395-9.
21. Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, et al. (2009) The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284:21257-64.
22. Zong C, Lu S, Chapman AR, Xie XS (2012) Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 338:1622-6.