Catheter-based intraluminal optical coherence tomography: comparison with digital light microscopy of ureter wall morphometry in porcine specimens
Main Article Content
Keywords
digital light microscopy, morphometry, optical coherence tomography, urinary tract, ureter
Abstract
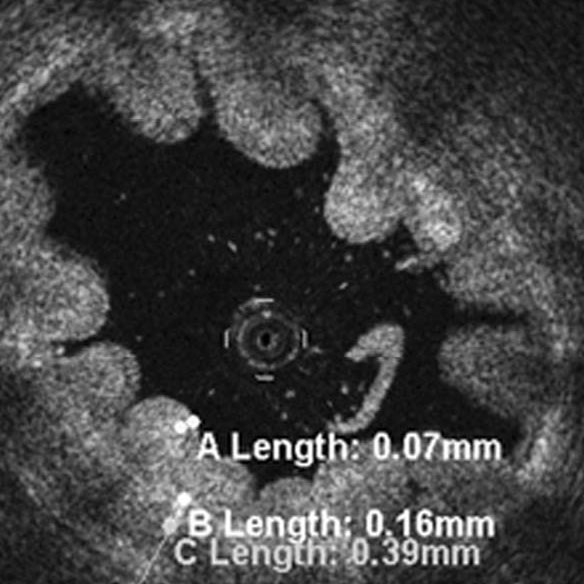
Objective: Reproducibility and agreement of width estimates for urothelium, lamina propria, and muscle layer of normal porcine ureter wall ex vivo were compared between catheter-mounted, intraluminal cross-sectional optical coherence tomography (OCT), as a new destruction-free optical imaging method that could be applied clinically to examine the upper urinary tract from within if found to reliably delineate its different anatomical layers, and whole-mount low-power digital light microscopy (DLM).
Materials and Methods: Eleven ex-vivo-specimens of porcine ureter were flushed with normal saline solution prior to OCT (catheter diameter, 0.014 inch, wavelength, 1300 ± 20 nm, LightLab Imaging, Inc., Westford, MA, USA) at marked locations. Ring-shaped (3 mm) ureter specimens were fixed in 4%-formalin, cut, whole-mounted, and hematoxilin-eosin-stained. Respective thicknesses of urothelium, lamina propria, and muscle layer of the ureter wall were compared between OCT and matching DLM slides (Axioplan2, Carl-Zeiss-Corporation, Jena, Germany) by two independent observers, applying Bland-Altmann plots and paired-data-Student-T-testing at P < 0.05.
Results: Width estimates were reproducibly higher for urothelium by a factor of about 1.4 at OCT (60 ± 9–96 ± 18 μm) compared to DLM (45 ± 12–68 ± 9 μm, P < 0.01), similar for lamina propria (OCT, 191 ± 21–233 ± 44 μm; DLM, 189 ± 48–326 ± 58 μm), and variable for muscle layer (OCT, 355 ± 109–631 ± 42 μm; DLM, 454 ± 97–527 ± 121 μm).
Conclusions: It appears that OCT reliably delineates ureter wall layers demonstrated by DLM, and that intra-observer reproducibility of the width of urothelium and lamina propria is high for both OCT and DLM, width estimates of lamina propria are reliable at OCT, and width of urothelium is reproducibly overestimated at OCT when compared with DLM.
Metrics
References
[2] Mueller-Lisse UL, Meissner OA, Babaryka G, Bauer M, Eibel R, et al. (2006) Catheter-based intraluminal optical coherence tomography (OCT) of the ureter: ex-vivo correlation with histology in porcine specimens. Eur Radiol 16: 2259-2264.
[3] Brezinski ME, Tearney GJ, Weissman NJ, Boppart SA, Bouma BE, et al. (1997) Assessing atherosclerotic plaque morphology: comparison of optical coherence tomography and high frequency intravascular ultrasound. Heart 77 397-403
[4] Fujimoto JG, Boppart SA, Tearney GJ, Bouma BE, Pitris C, Brezinski ME (1999) High resolution in vivo intra-arterial imaging with optical coherence tomography. Heart 82: 128-133.
[5] Tearney GJ, Brezinski ME, Southern JF, Bouma BE, Boppart SA, Fujimoto JG (1997) Optical biopsy in human gastrointestinal tissue using optical coherence tomography. Am J Gastroenterol 92: 1777-1779.
[6] Meissner OA, Rieber J, Babaryka G, Oswald M, Reim S, et al. (2006) Intravascular optical coherence tomography: comparison with histopathology in atherosclerotic peripheral artery specimens. J Vasc Interv Radiol 17: 343-349.
[7] Boppart SA, Bouma BE, Pitris C, Tearney GJ, Southern JF, et al. (1998) Intraoperative assessment of microsurgery with three-dimensional optical coherence tomography. Radiology 208: 81-86.
[8] Jesser CA, Boppart SA, Pitris C, Stamper DL, Nielsen GP, et al. (1999) High resolution imaging of transitional cell carcinoma with optical coherence tomography: feasibility for the evaluation of bladder pathology. Br J Radiol 72: 1170-1176.
[9] Zagaynova EV, Streltsova OS, Gladkova ND, Snopova LB, Gelikonov GV, et al. (2002) In vivo optical coherence tomography: feasibility for bladder disease. J Urol 167: 1492-1496.
[10] Pan YT, Xie TQ, Du CW, Bastacky S, Meyer S, Zeidel ML (2003) Enhancing early bladder cancer detection with fluorescence-guided endoscopic optical coherence tomography. Opt Lett 28: 2485-2487.
[11] Glantz SA (1997) Comparing two different measurements of the same thing: the Bland-Altmann method. In: Glantz SA. Primer of Biostatistics. Fourth Edition. Chapter 8: How to test for trends. New York, St. Louis, San Francisco, Auckland, Bogota, Caracas, Lisbon, London, Madrid, Mexico City, Milan, Montreal, New Delhi, San Juan, Sydney, Tokyo, Toronto: McGraw-Hill Health Professions Division. pp 266-271.
[12] Glantz SA (1997) Experiments when subjects are observed before and after a single treatment: the paired t test. In: Glantz SA. Primer of Biostatistics. Fourth Edition. Chapter 9: Experiments when each subject receives more than one treatment. New York, St. Louis, San Francisco, Auckland, Bogota, Caracas, Lisbon, London, Madrid, Mexico City, Milan, Montreal, New Delhi, San Juan, Sydney, Tokyo, Toronto: McGraw-Hill Health Professions Division. pp 283-291.
[13] Desgrandchamps F, Moulinier F, Cochand-Priollet B, Wassef M, Teillac P, Le Duc A (1997) Microscopic study of the pig ureteral urothelium. J Urol 157: 1926-1927.
[14] Leeson CR, Leeson TS, Paparo AA (1985) The preparation of tissues. In: Leeson CR, Leeson TS, Paparo AA. Textbook of Histology. Introduction. Philadelphia, London, Toronto, Mexico City, Rio de Janeiro, Sydney, Tokyo: WB Saunders Company. pp 5-10.
[15] Leeson CR, Leeson TS, Paparo AA (1985) Excretory Passages. In: Leeson CR, Leeson TS, Paparo AA. Textbook of Histology. Part II. Histology of the organ systems. Chapter 13. The urinary system. Philadelphia, London, Toronto, Mexico City, Rio de Janeiro, Sydney, Tokyo: WB Saunders Company. pp 432-434.
[16] Siegel RJ, Swan K, Edwalds G, Fishbein MC (1985) Limitations of postmortem assessment of human coronary artery size and luminal narrowing: differential effects of tissue fixation and processing on vessels with different degrees of atherosclerosis. J Am Coll Cardiol 5: 342-346.